(25
July, 2023, Shanghai, China)Shanghai
Fosun Pharmaceutical (Group) Co., Ltd (“Fosun Pharma”; stock code: 600196.SH, 02196.HK)
announced that the MEK1/2 inhibitor FCN-159 tablet (the“Investigational New Drug”) for the treatment
for adult patients with inoperable or post-operative residual/recurrent
NF1-associated plexiform neurofibromas, which was self-developed by its holding
subsidiary Shanghai Fosun Pharmaceutical Industrial Development Co., Ltd.
("Fosun Pharma Industrial"), have been granted breakthrough therapy designation
by the Centre for Drug Evaluation (CDE) of National Medical Products
Administration (the “NMPA”).This is the second breakthrough therapy
designation that FCN-159 tablet has approved, since its indication for histiocytic
tumor in April this year.
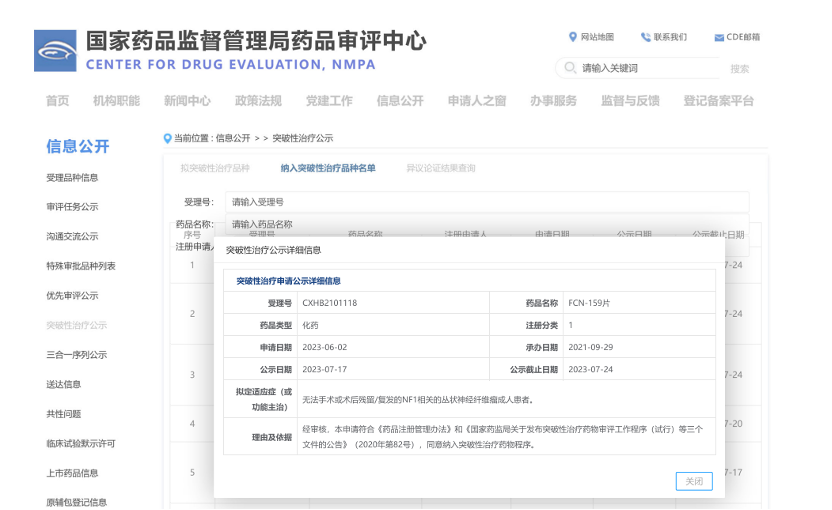
(website
of the CDE.)
Neurofibromatosis
1 (NF1) is an autosomal dominant disorder characterized by enhanced
RAS-mitogen-activated protein kinase (RAS-MAPK) signaling pathway, leading the
tumors to grow along nerves. 20%-50% of NF1 cases present as plexiform
neurofibromas (PN), which may result in complications including pain,
functional impairment, disfigurement and malignant transformation[1].Surgery
is currently the mainstay treatment for neurofibromatosiswith limited efficacy[1].
However, there is no approved drugs for adult participants so far.
In
June, the results from phase I/II study of FCN-159 in adult participants with
neurofibromatosis type 1 were presented at the 2023 American Society of
Clinical Oncology (ASCO) Annual Meeting. The overall response rate (ORR) was
45.1% and had a manageable safety profile in the adult population with
NF1-related PN. Meanwhile, the phase I results of FCN-159 in adult participants
with neurofibromatosis type 1 were published in BMC Medicine (2023 impact
factor 9.3), and the results showed that FCN-159 was well tolerated and adverse
events were manageable. It also showed promising antitumor activity in
NF-1-associated PN patients, Future investigations are warranted. The Phase III
clinical study is currently underway.
(The
figure shows the original publication on BMC Medicine.)
FCN-159
tablet is an innovative small molecule chemical drug self-developed by Fosun
Pharma, which is intended to be used primarily for the treatment of advanced
solid tumors, eurofibromatosis type 1, histiocytic neoplasms and arteriovenous
malformations. It is a MEK1/2 selective inhibitor that can inhibit tumor
proliferation caused by abnormal RAS pathway. The Investigational New Drug is
at the stage of Phase III clinical trial in China (excluding Hong Kong, Macao
and Taiwan region, the same applies below) for the treatment of type I
neurofibroma in adults, and it is still at the stage of Phase II clinical trial
in China, the United States and Europe’s International Multi-Centers for the
treatment of type I neurofibroma in adults and children. The Investigational
New Drug is at the stage of Phase II clinical trials in China for the treatment
of histiocytic tumors, low-grade glioma and arteriovenous malformations
respectively. It has also been granted breakthrough therapy designation for the
treatment of histiocytic tumors. The application for Phase II clinical trial of
the Investigational New Drug for childhood Langerhans cell
histiocytosis/Langerhans cell histiocytic hyperplasia has been approved by the
NMPA.
References
1. Jett
K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1.Genet
Med 2010; 12:1-11..
2. Hu X,
et al. Phase 1 dose-escalation study to evaluate the safety, tolerability,
pharmacokinetics, and anti-tumor activity of FCN-159 in adults with
neurofibromatosis type 1-related unresectable plexiform neurofibromas.BMC
Med. 2023 Jul 3;21(1):230. doi: 10.1186/s12916-023-02927-2.
About the Breakthrough Therapy Designation
To
encourage the research and development of drugs with significant clinical
advantages, the Center for Drug Evaluation (CDE) of the National Medical
Products Administration (NMPA) has issued document on《NPMA announcement about issuing three documents
including 》(No. 82 of 2020), which clarifies the included scope
of breakthrough therapy designation: during drug clinical trial, innovative
drugs or improved new drugs used for the prevention and treatment of diseases
that are seriously life-threatening or seriously affect the quality of life
,and have no effective prevention and treatment or have sufficient evidence to
show obvious clinical advantages compared with existing treatment. CDE will prioritize
resources of the drugs that have been included in the breakthrough therapy designation
and communicate, enhance guidance and promote drug development.
About
Fosun Pharma
Founded
in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd.* ("Fosun
Pharma"; stock code: 600196. SH, 02196. HK) is a global innovation-driven
pharmaceutical and healthcare industry group. Fosun Pharma directly operates
businesses including pharmaceuticals, medical devices, medical diagnosis, and
healthcare services. As a shareholder of Sinopharm Co., Ltd., Fosun Pharma
expands its areas in the pharmaceutical distribution and retail business.
Fosun
Pharma is patient-centered and clinical needs-oriented. The company continuously
enriches its innovative product pipeline through independent research and
development, cooperative development, license-in, and in-depth incubation.
Fosun Pharma improves the research and clinical development capabilities of FIC
(First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the
R&D and launch of innovative technologies and products.
Guided
by the 4IN strategy (Innovation, Internationalization, Intelligentization, and
Integration), Fosun Pharma will uphold the development model of “Innovation
Transformation, Integrated Operation and Steady Growth", with the mission
of creating shareholder values through strengthening its independent R&D
and external cooperation and enriching its product pipelines, as well as promoting
the global networks and enhancing operational efficiency. Fosun Pharma will
actively promote the digital and physical business layout in the pharmaceutical
and healthcare industry and is committed to becoming a first-class enterprise
in the global pharmaceutical and healthcare markets.
For
more information, please visit our official website:www.fxcprm.com